How Are Bright Line Spectra Produced by Atoms
When solids liquids or condensed gases are heated sufficiently they radiate some of the excess energy as light. Which elements are present in this mixture.

Formation Of Spectral Lines Astronomy
Continuous spectra are produced by electrons being shared between many atoms giving a.

. When electrons jump from the excited state to the ground state the electrons emit energy in the form of light producing a bright-line spectrum. Another paradox within the classical electromagnetic theory that scientists in the late nineteenth century struggled with concerned the light emitted from atoms and molecules. Kirchhoffs Laws are.
Click to see full answer. This means that line spectra can be used to identify elements. Photons produced in this manner have a range of energies and thereby.
The bright spectrum is produced by the electrons in the elements atoms jumping to lower energy states after being bumped upward by a. Accurately identify the presence of an element in an unknown mixture by comparing the emission spectrum of a mixture with the. A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum resulting from emission or absorption of light in a narrow frequency range compared with the nearby frequencies.
A hot gas under low pressure produces a bright-line or emission line spectrum. Spectral lines are often used to identify atoms and molecules. Emission Line Spectrum The atom is first excited by a colliding electron.
So they are used to identify the atomic and molecular components of stars and planets which would otherwise be impossible. Astronomers and physicists have worked hard to learn the lines that go with each element by studying the way atoms absorb and emit light in laboratories here on Earth. Atoms of individual elements emit light at only specific wavelengths producing a line spectrum rather than the continuous spectrum of all wavelengths produced by a hot object.
This means that each type of atom shows its own unique set of spectral lines produced by electrons moving between its unique set of orbits. Niels Bohr explained the line spectrum of the hydrogen atom by assuming that the electron moved in circular orbits and that orbits with only certain radii were allowed. For instance there are many different mechanisms by which an object like a star can produce light.
2 Elements A and D are present in the mixture. The energy levels in the atom have definite values. Line spectra are produced when electrons move from one energy level within an atom to another energy level.
Spectra can be produced for any energy of light from low-energy radio waves to very high-energy gamma rays. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Each spectrum holds a wide variety of information.
This pattern like a fingerprint is unique to that element. When the atom goes back to its ground state either directly or via intermediate energy levels photon of only certain frequencies are emitted due to the discrete energy levels. The bright-line spectra produced by four elements are represented in the diagram below.
2 The two lines at 585 indicate the presence of sodium Na. A hot solid liquid or gas under high pressure gives off a continuous spectrum. The emission lines correspond to photons of discrete energies that are emitted when excited atomic states in the gas make transitions back to lower-lying levels.
Given the bright-line spectrum of a mixture formed from two of these elements. A continuum spectrum results when the gas pressures are higher so that lines are broadened by collisions between the atoms until they are smeared into a continuum. Qualitatively relate the energy of electronic transitions to the specific colors of light observed.
Each of these mechanisms has a characteristic spectrum. Each element has its own unique bright-line. Bright Line Spectra A pattern of lines produced by using a prism to dissect the light given off by an excited atom.
These fingerprints can be compared to the previously collected ones of atoms and molecules and are thus used. The wavelength of the emission or absorption lines depends on what atoms are molecules are found in the object under study. When a narrow beam of this light was viewed through a prism the light was separated into four lines of very specific wavelengths and frequencies since and are inversely related.
This video lesson covers Bright Line Emission Spectra how electrons get excited how electrons emit light how fireworks colors are formed and how we can u. Describe how a bright line spectrum is produced in terms of energy transitions of the electrons in an atom as they move from one energy level to another. We may view a continuum spectrum as an.
If the atom is emitting light then the spectral line is bright if the atom is absorbing light the spectral line is dark So emission spectra are bright.
Lines Spectra And Excited Electron States
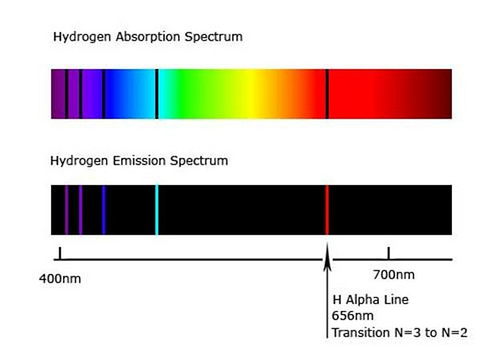
Absorption Spectrum Emission Spectrum Lines Article Khan Academy
Comments
Post a Comment